Search results of guideline publications
The search returned 25 articles from Medline. After reading the title and abstract of each article, none of them were considered a potentially relevant article regarding the informatics system design in the field.
The search identified 23 documents by Google and Google Scholar, and the experts suggested two extra documents potentially relevant. Most of them were white paper or industrial documentation. The majority of them were lacking of explanations with respect to the validation process. After skimming the full documents, low reliable articles were excluded. They were either individual’s rules of thumb or reorganization of established guidelines based on the trustworthy sources. Eventually, four documents were selected to retrieve guideline items. They were from four reliable sources of Nielsen Norman Group, Department of Health and Human Services (DHHS), NIST-7865 and Microsoft Common User Interface (CUI).
Table 1, general features of qualified guideline documents
|
Publish year |
Title |
Author | Descriptive granularity |
Domain |
Volume |
Methods of development |
|
2001 |
113 Design Guidelines for Homepage Usability [1] | Nielsen | Low |
General |
26 categories 113 guidelines | Empirical study based |
|
2006 |
The Research-Based Web Design & Usability Guidelines [2] | DHHS | Low |
General |
209 guidelines | Expert review |
|
2012 |
Microsoft Health Common User Interface Guidelines [3] | Microsoft | High and low |
Health |
33 categories with thousands guidelines | Expert review |
|
2012 |
A Human Factors Guide to Enhance EHR Usability of Critical User Interactions when Supporting Pediatric Patient Care [4] | NIST | High and low |
Health |
9 categories of recommendations | Expert review |
Matching results on usability principles
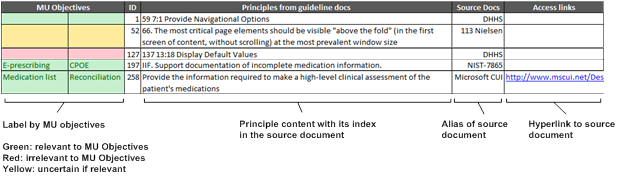
All principles from four document sources were tabulated in a format as shown in Figure 1. In the table, they were characterized mainly by five aspects of MU objectives, sequence ID, principle summary, source document and access hyperlinks. The numbered parts of principle summary are internal index in the document that helps locate related descriptors. As for the records without such index such as the principles from Microsoft CUI, the hyperlink of the specific piece of the document provides for easy access.
During the matching process, the curators had two tasks. One was to justify the relevance of the principle to EHR design with color codes. Green meant the principle was applicable; red meant inapplicable and yellow was uncertain. The second task was to match the principle up with MU objectives one by one. Two most appropriate MU objectives were labeled with the record in the table. Furthermore, each record was duplicated into additional spreadsheets by MU objectives. If one record could fit in more than four MU objectives, it eventually was put into an independent category as a general principle. Label inconsistency was resolved on a basis of group discussion.
Figure 1, the table structure of compiled guidance principles
In the end, 303 principles were identified as applicable to the design of EHR and 133 of them were general. As for the rest 170 principles, they were designated to eight categories of MU objectives as shown below. Although we tried to assign principles to the categories exclusively, around half of them were cross labeled. Table 2 shows the distribution of principles by MU objectives, and Table 3 using e-prescribing as an example illustrating how assigned principles to be organized under each category of MU objectives.
Table 2, the distribution of principles by MU objectives
|
MU objectives |
# of principles that fitted into this category |
| Medication list |
8 |
| Drug-drug and drug allergy interaction checks |
25 |
| Medication allergy list |
25 |
| E-prescribing |
55 |
| CPOE |
64 |
| Clinical decision support, |
12 |
| Electronic medication administration record |
13 |
| Clinical information reconciliation |
45 |
As shown in Table 3, the majority of principles has numbered parts under the column of principles. They are locators for retrieving the principle details in the corresponding source document. As for the principles extracted from Microsoft CUI in which this information is not available, we attached the hyperlink to each of them that indicates in which specific file the principle was found. To interpret number parts of principles from the other three source documents, the rules are listed as below:
Table 3, the aggregation of guidelines with respect to e-prescribing
|
ID |
Principles |
Source Docs |
| 113 | 121 13:1 Distinguish required and optional data entry fields |
DHHS |
| 114 | 123 13:3 Label data entry fields consistently |
DHHS |
| 115 | 123 13:4 Do not make user-entered codes case sensitive |
DHHS |
| 116 | 124 13:5 Label data entry fields clearly |
DHHS |
| 117 | 125 13:6 Minimize user data entry |
DHHS |
| 118 | 126 13:7 Put labels close to data entry fields |
DHHS |
| 119 | 127 13:8 Allow users to see their entered data |
DHHS |
| 120 | 128 13:9 Use radio buttons for mutually exclusive selections |
DHHS |
| 121 | 130 13:11 Anticipate typical user errors |
DHHS |
| 122 | 131 13:12 Partition long data items |
DHHS |
| 124 | 134 13:15 Use check boxes to enable multiple selections |
DHHS |
| 125 | 135 13:16 Label units of measurement |
DHHS |
| 128 | 138 13:19 Place cursor in first data entry field |
DHHS |
| 130 | 140 13:22 Use data entry fields to speed performance |
DHHS |
| 131 | 140 13:23 Use a minimum of two radio buttons |
DHHS |
| 135 | 71. Use dropdown menus sparingly, especially if the items in them are not self-explanatory |
113 Nielsen |
| 136 | 24. Only use imperative language such as “Enter a City or Zip Code” for mandatory tasks, or qualify the statement appropriately |
113 Nielsen |
| 192 | IIA. Protect against mode errors for mg/kg dosing and ml dosing. |
NIST-7865 |
| 193 | IIB. Flag that an intended dose is unusual. |
NIST-7865 |
| 194 | IIC. Support high-precision dosing for low-weight patients. |
NIST-7865 |
| 195 | IID. Do not permit automated defaults to adult doses. |
NIST-7865 |
| 196 | IIE. Support custom formulations for liquid medications. |
NIST-7865 |
| 197 | IIF. Support documentation of incomplete medication information. |
NIST-7865 |
| 198 | IIG. Reduce displayed options for medication orders. |
NIST-7865 |
| 199 | IIH. Display the recommended dose range for the selected mg/kg dose. |
NIST-7865 |
| 200 | III. Display “input masks” for data entry to clarify type of data. |
NIST-7865 |
| 201 | IIJ. Avoid truncation of medication names and dosages in menus. |
NIST-7865 |
| 202 | IIIA. Support flexibility in unit-based settings for alerts, reminders, and warnings based upon weight, height, Body Surface Area, Body Mass Index, and age. |
NIST-7865 |
| 203 | IIIB. Do not permit replacing pediatric-specific thresholds with default adult-based thresholds following a system-wide crash. |
NIST-7865 |
| 204 | IIIC. Do not permit “hard stops” for changes to medication orders. |
NIST-7865 |
| 205 | IIID. Cap the dose at the standard adult dose and allow override with justification. |
NIST-7865 |
| 206 | IIIE. Display normal ranges for medication doses and lab values based upon weight, height, Body Surface Area, Body Mass Index, and age information. |
NIST-7865 |
| 207 | IIIF. Display together parameters that are continuously monitored to rapidly intervene. |
NIST-7865 |
| 242 | Highlight the primary functions to support accurate recording of what happened for an administration event |
Microsoft CUI |
| 243 | Display safety critical elements to the clinician without requiring user action |
Microsoft CUI |
| 244 | Promote the primary functions to support quick recording of an administration event |
Microsoft CUI |
| 245 | Support access to secondary functions without introducing screen clutter |
Microsoft CUI |
| 246 | Transfer key design principles from the paper drug charts studied to reduce the need for training and increase familiarity when users move to electronic systems. |
Microsoft CUI |
| 247 | Provide a visually-rich chart of information relevant to, and prioritized for, the administration of drugs |
Microsoft CUI |
| 248 | Support the presentation of drugs with different characteristics (such as Significant Duration, Once Only or As Required drugs) within one view |
Microsoft CUI |
| 249 | Display sufficient information for an accurate interpretation of the administration schedule(past, current and planned) and status of administration events within a relevant time interval |
Microsoft CUI |
| 250 | Restrict the display of unnecessary information to reduce clutter and prioritize the information most likely to require action |
Microsoft CUI |
| 251 | Provide access, in context, to further details on demand |
Microsoft CUI |
| 252 | Mitigate the potential for action to be taken without sufficient information by presenting carefully selected information and explicit labels to clarify what information is displayed and the extent to which it is complete |
Microsoft CUI |
| 253 | When dynamically presenting information (such as status information, error messages o warnings), display the information in context and facilitate action where necessary by clearly providing associated controls |
Microsoft CUI |
| 254 | Support efficient and accurate recording of administration events with enough flexibility for differences in drugs and working practices |
Microsoft CUI |
| 263 | Mitigate the risks of mis-selection and misinterpretation |
Microsoft CUI |
| 264 | Increase efficiency by prioritizing the prescription of commonly prescribed medications over less commonly prescribed medications |
Microsoft CUI |
| 265 | Maximize safety in the absence of decision support systems by designing for the reduction of errors from invalid or inappropriate selections or entries |
Microsoft CUI |
| 266 | Encourage simplicity of design by promoting user interface approaches that help to avoid overly complex displays and interactions that require many controls |
Microsoft CUI |
| 267 | Ensure that the prescribing process can be supported in multiple layouts and is flexible enough to be presented in different screen dimensions |
Microsoft CUI |
| 268 | Maximize scalability such that the prescribing process can be modified to accommodate additional information, steps or shortcuts |
Microsoft CUI |
| 269 | Manage users expectations and improve their efficiency by providing a clear framework with consistent logic for the placement of user interface elements and the interactions that they support |
Microsoft CUI |
| 270 | Minimize the potential for important information to be hidden from view |
Microsoft CUI |
| 271 | Adhere to a user interface strategy that gives the impression of making progress within a single space (that has all the necessary information immediately or readily available) and avoids the impression of needing to move between many different spaces |
Microsoft CUI |