The Clinical Data Warehouse is intended to provide UTHealth investigators with a single source for obtaining access to vast amounts of clinical data available in various systems. The data can be used for multiple research tasks including preparatory research, cohort identification, and data mining on clinical records. In addition to research, the system may also be used for Quality Improvement projects. As a member of the CCTS consortium, BIG’s goal is to help develop standardized processes for Comparative Effectiveness, Quality Improvement, and Drug Safety Research that will ensure compliance with regulatory requirements associated with accessing ePHI data.
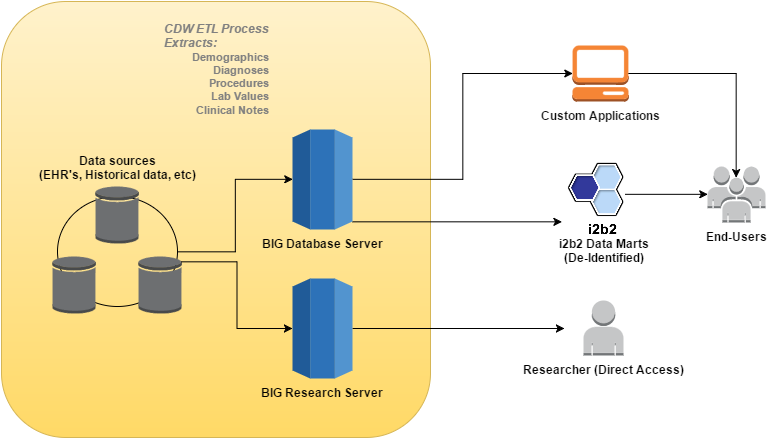
i2b2 is a project sponsored by the NIH Roadmap National Centers for Biomedical Computing. i2b2 software is the backbone of our CDW and serves two important purposes:
Together, these tasks promote the rapid development of research projects while protecting Personal Health Information.
Click here for summarized statistics on the data we collect.
Currently, information from UT Physician's outpatient practice (currently Epic and previously Allscripts), historical UT Physicians billing database (GECBI), UTHealth School of Dentistry's billing and imaging data (AXIUM), and select data elements from several Memorial Hermann Hospital sources is available.
The CDW also integrates information through several data sharing partnerships, such as the National COVID Cohort Collaborative (N3C), the Greater Plains Collaborative (GPC), and the Accrual to Clinical Trials (ACT) Network. In addition, data is incorporated from affiliate schools, such as University of Texas at Tyler.
Researchers interested in using CDW data for a clinical study will first need to complete a Request For Data form. The request will be sent to the CCTS Technology Manager for review; if the request is research oriented and involves PHI, IRB approval will be needed. After approval, the CCTS technology team will begin processing the data request, communicating preliminary results to the researcher(s) to facilitate data quality and validation assessments. The CCTS IT Staff provide follow-up support to researchers on issues such as data management and use.
Read Access to Clinical Data for a description of the request process and the related HIPAA requirements.